This paper, written by seventeen year old Adam Michaelson, has been entered into the Intel Science Talent Search in the USA, and we are, with Adam's permission, reproducing it here. Read on!
Behavioral and Attitudinal Effects of
Youth and General Onset
Parkinsonís Disease
By
Adam D. Michaelson
Oceanside High School, Oceanside, New York
RESEARCH REPORT FOR THE
INTEL SCIENCE TALENT SEARCH
Summary
A
sample of 78 people with Parkinsonís Disease was analyzed to examine effects
in
their daily lives.† The data was
collected through questionnaires distributed in the USA and Ireland.† The main findings demonstrate that there is
a fine distinction between Youth Onset Parkinsonís Disease, or diagnosis before
fifty years of age, and General Onset Parkinsonís Disease, or diagnosis after
fifty years of age.† The data shows that
people with Youth Onset Parkinsonís Disease experience a faster progression of
the disease, more severe psychological effects, and a tendency toward rejection
of medications. Because of the information collected, health care providers
will learn how better to treat both types of Parkinsonís Disease and how to
optimize patientís quality of life. †
†††††††††
Parkinsonís Disease affects more people than do Multiple Sclerosis and
Muscular Dystrophy.† It strikes with no
warning signs and at any age.† This
disease is much different from Alzheimerís in that the sufferer is fully aware
of the progression of the disease and all the disabilities that come with
it.†
††††††††† The disease is classified
as a neuromuscular disorder resulting from the degeneration of pigmented
neurons in two areas of the brain: the substantia nigra and the locus
ceruleus.† The disease is detected by
the presence of Lewy bodies in these regions.†
Lewy bodies are only present when pigmented neurons are dying or dead.
There are many types of rarely occurring Parkinson Diseases that will not be
examined in this study in order to discuss the effects of the disease on the
majority of the population with the disease.†
The following variations of Parkinsonís Disease will be excluded from
this study: Vascular Parkinsonism or Parkinsonian symptoms resulting from a
lack of oxygen and stroke, Infectious Parkinsonism or virus induced Parkinsonís
Disease (like encephalitis lethargica), Drug-Induced Parkinsonism or symptoms
resulting from persistent use of early tranquilizers like resperine,
haloperidol, and chlorpromazine, and any of the diseases classified as
Parkinsonís Syndrome (including chemical poison induced Parkinsonism and
Pugilistic Parkinsonism) (Lieberman and Williams 1993). This study will only
deal with the most common form of Parkinsonís Disease: Idiopathic Parkinsonís
Disease. The remainder of this study will refer to Idiopathic Parkinsonís
Disease as Parkinsonís Disease.
††††††††† This research is geared toward the dissection of
Parkinsonís Disease. It will be compared to the same disease in another
country, its effect on the quality of life of the sufferer, and the difference
age of onset has on the disease.† The
study relates certain aspects of the disease such as psychological effects,
sleep disturbances, cognitive degeneration, rate of progression, contributing
factors, quality of life, and medicinal side effects.
Since the study only examines the effects of the
disease and not the physical degeneration of the dopamine in the brain,
progression does not describe the disease in its natural state, but describes
how the medicine and the disease progress together (Furukawa, Mizuno, and
Narabayasi).† Also, progression of the disease is classified by
symptoms and not by the decline in F-dopamine uptake in the brainís striatal
subregion because of the recent study done at the University of Turku (Nurmi et
al. 2001.
††††††††††† With the sustained efforts of scientists using stem
cells, an end to Parkinsonís Disease looks promising.† However, until conclusive biological findings are made, social
scientists must find out how to help the psyche of the Parkinsonís Disease
patient.† If used correctly, the
information attained from this study can increase the quality of life of
patients. Certain distinctions will be made between Youth Onset Parkinsonís
Disease (YOPD) and General Onset Parkinsonís Disease throughout the research.
These distinctions may become instrumental in the medical as well as holistic
treatment of each disease.
Literature
Review
†††††††††
An intensive literature review was conducted in order to determine what
information is available to both the Parkinsonís Disease patient and the
researcher. Medical journals, Internet forums, and health care pamphlets were
most useful throughout this study. It has been very important to understand the
studies conducted by other researchers.
Quality of
Life
In order to prove that
Youth Onset Parkinsonís Disease and General Onset Parkinsonís Disease are
different, the statistics must show that each disease affects the patient in a
different way.† Without using any
biological changes in the body for justification, the optimal mode of achieving
this goal is through comparing the quality of life of each group of
respondents.† An overall hypothesis must
be drawn that states that one condition of the disease brings about a lower
quality of life than another.† Quality
of life is not a measurable variable.†
However, ≠Schrag,
Jahuppshahi, and Quinn have stated that depression, disability, disease
severity, and cognitive impairment account for the poorer quality of life in PD
sufferers.† Upon analyzing this studyís
data and interviewing Parkinsonís Disease patients, many psychological
illnesses appear to accompany the disease. A fifth component appears necessary
to compare the two groups.† This fifth
component tests the psychological effects the disease has on the
respondent.† These effects include
anxiety, paranoia, agoraphobia, and hallucination.† In order to quantify the collected data, several individual
indices have been created.† Only
physical disability will be tested so as not to double count data.†
The five components thus
are Disease Severity, Degree of Depression, Physical Disability, Cognitive
Impairment, and Psychological Effects.†
These five components are based on a zero to one scale where zero is
most affected by the component and one is least affected by the component.† The sum of the five components represents
the Quality of Life Index.† This can
range from zero to five, where minimum quality of life is zero and maximum
quality of life is five.† Each component
has been evaluated independently as well.
Another indicator of the
difference between YOPD and General Onset Parkinsonís Disease is found in the
different of levelsof independence that the
respondents feel.† An index was created
to establish the degree to which the two subgroups were dependent upon a caregiver
or relative where zero is independent and five is totally dependent. This test
is useful in describing the difference in a change in lifestyle of respondents
in each group.
†††††††††
The questionnaire was limited to general issues of physical symptoms and
observances rather than beliefs. Since General Onset Parkinsonís Disease is
limited to geriatrics, it is difficult to discern between the effects of aging
and the effects of the disease.† For
future comparisons between Youth Onset Parkinsonís Disease and General Onset
Parkinsonís Disease, a control group must be used for the measurement of
cognitive impairment. According to all the information accessible, no previous
studies have been done to compare YOPD to General Onset Parkinsonís Disease holistically.† Because of this, the statistics and analysis
of much of the data in this study cannot be compared to other studies with the
same hypotheses.† Also, the distribution
of respondents across the Hoehn and Yahr Scale is not even.† Because Stage IV and Stage V patients
experience a lack of motility and cognitive reasoning, answering a
questionnaire is very difficult.† Thus,
fewer questionnaires were received from this group than any other.† For future studies, an equal number of
respondents from each stage should be important in comparing these stages.
Based on the information acquired from interviews with patients, talks
with Movement Disorder Specialists and current research in Parkinsonís Disease,
the following hypotheses have been proposed for analysis.
1)
For the two-tailed test, the following comparisons were made:
∑
H0 There is no significant
difference between the mean rate of progression of Parkinsonís Disease patients
from the United States and Parkinsonís Disease patients from Ireland.
H1The mean rate
of progression is greater in Ireland than in the United States.
∑
H0 The frequency of YOPD
respondents and General Onset Parkinsonís Disease respondents experiencing
denial of the diseaseís severity is equal.
H1 The frequency
of YOPD respondents experiencing denial is greater than the frequency of
General Onset Parkinsonís Disease respondents.
∑
H0 The mean Quality of Life
Index is the same in YOPD and General Onset Parkinsonís Disease respondents.
†††† H1 The mean Quality of Life Index is higher in
General Onset Parkinsonís Disease
respondents
than in YOPD respondents.
†††††††††
The main research tool used in this study was a questionnaire.† Two preliminary studies were conducted for
the efficacy of the questions before the final version was produced.† Contacts were provided through support
groups both online and in person.†
Questionnaires sent to Ireland were distributed by a support group
leader and then returned by mail.† The
patients were asked neither their names nor geographic locations (excluding
country) in order to ensure anonymity.†
Most questionnaires were sent with cover letters with self
addressed-stamped envelopes.† About
fifteen of the questionnaires were sent via e-mail to only people who requested
it and were members of the support group forum.† Upon return, the envelopes were discarded and each questionnaire
was assigned a sequential number.
††††††††† The questionnaire was
designed to investigate certain aspects of the disease. It asked for both
subjective and objective answers.† Most
questions were multiple choice with room for explanation and ďotherĒ answers
where applicable.†
The makeup of the
questionnaire was not based on any previously distributed questionnaires.† Respondents were unaware of the researcherís
intentions to find distinctions in the onset of the disease.† Without this bias, many of the answers
respondents gave were consistent.† Many
previous studies have investigated differences in onset of Parkinsonís Disease
to various factors of the disease.† But,
despite intensive research, no previous study has tried to differentiate the
two diseases as distinct.
††††††††† The data was analyzed
with a TI-83 plus calculator and Microsoft Excel.† Descriptive statistics was used to show differences in mean,
standard deviation, and frequency distribution.† For inferences, the two-tailed independent sample t-test and a two-sample t test statistic were used to evaluate
the hypotheses.
One hundred
questionnaires were distributed with an unusually high response rate (84%) of
84 questionnaires returned.† Five
surveys were disqualified from statistical analysis because they lacked answers
to questions that were necessary in correlating data.† One survey was also disqualified since the respondent
simultaneously was a part of a New York University drug study.† In effect, his answers to the questionnaire
would be inaccurate whether he used his present conditions from the unknown
drug or inconclusive because of uncertainty about symptoms before the
study.† As a result, seventy-eight cases
were available for statistical analysis.
The sample population was
drawn from the United States and Ireland.†
The respondents came from random parts of both countries to further
ensure anonymity and geographic predisposition.†
The differences between
the rates of progression for the subgroups of American respondents and Irish
respondents were tested with the independent sample two-tailed t-test.†
The two-tailed test with an acceptance level of .05 was selected because
the direction of the correlation was not known.† As a result, the null hypothesis for rates of progression of both
subgroups was accepted, because there was no significant difference found in
the rates of progression.† Therefore,
the rate of progression of Parkinsonís Disease is not affected by geographic
location.† This test suggests that the
American respondents and Irish respondents come from the same population of
people with Parkinsonís Disease and will be used as such for the remainder of
the study.
The sample consists of 34
respondents with onset equal to or younger than 50 (or YOPD respondents) and 44
respondents with onset greater than 50 (or General Onset Parkinsonís Disease
respondents).† The most useful explicit
information that respondents offered is the respondentsí rating on the Hoehn
and Yahr Scale.
Table 1. The Hoehn and
Yahr Scale
|
Stage I:
|
Symptoms are found only on one side of the body.
|
|
Stage II:
|
Symptoms are found on both sides of the body; walking and posture are
affected.
|
|
Stage III:
|
The ability to walk is impaired and there is slowing of body
movements.
|
|
Stage IV:
|
Severe symptoms include marked impairment in walking.
|
|
Stage V:
|
Complete immobility
|
(Schrag, Jahuppshahi, and Quinn 2000)
The
respondents were distributed among the five stages as shown below.
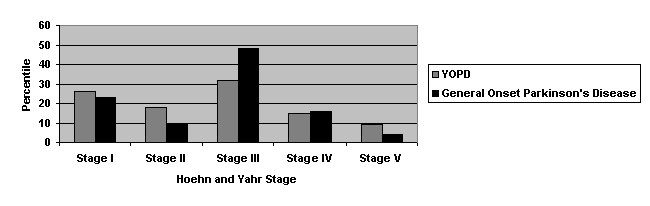
Table 2. Youth Onset
Parkinsonís Disease Respondents and General Onset Parkinsonís Disease
†††††††††††† Respondents By Hoehn and Yahr
Stage
†††††††††††††† †††
The distribution of the
respondents is indicative of the population of people with Parkinsonís Disease
that can successfully write or dictate answers to the questionnaire.† As the disease becomes more advanced, the
ability to perform normal tasks is hindered.†
This includes filling out a questionnaire.
†
Upon understanding the
stage distribution of the respondents, many factors become apparent. The
sleeping habits in YOPD respondents and General Onset PD respondents are
affected by stage.† While 100% of YOPD
respondents beyond Stage I experience some form of sleeping disorder, only 63%
of General Onset Parkinsonís respondents beyond Stage I recorded sleeping
problems.† Poor sleeping habits and
sleeping disorders can be attributed to either the disease itself or
medications that treat the disease. No conclusion can be drawn from the
questionnaires stating which reason is correct.† Since one or both of the attributions would cause the difference
in respondents with sleeping orders, two observations can be made. Between the
two groups, either the disease itself is different or the same medication
affects the two groups differently.†
Regardless of which hypothesis is true, either one indicates a marked
disparity between the two diseases.
Another observation also
became apparent when the two groups were split up into Hoehn and Yahr
Stages.† Several times throughout the
questionnaire respondents were given the opportunity to further support or
contradict previous answers.† The most
apparent and frequent contradictions came when General Onset Parkinsonís
Disease patients beyond Stage II were asked about their independence.† Fourteen of the thirty General Onset
respondents beyond Stage II described themselves as being ďcompletely
independent, so much that (they) can live alone.Ē† Of those fourteen patients, ten responded that they are dependent
on a caregiver or family member for much of their daily activities.† Only three of the thirty-four YOPD
respondents answered in the same manner.†
This inconsistency came as a surprise.
A two-tailed independent
sample t-test with a 95% confidence
level was used to evaluate the second hypothesis. A critical value of Ī2.145 was
used.† The t value wasĖ2.460 and thus the H≠≠0 was rejected and the
negation of the H1 was accepted. This test shows that less than 5%
of all samples will show a smaller discrepancy in dependence on others.† It can be concluded that General Onset
Parkinsonís Disease patients suffer from denial and problems with self-identity
more so than the YOPD patient.
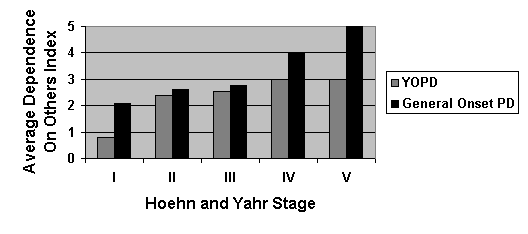
The Hoehn and Yahr Scale
shows a difference in the average degree of dependence the respondents have on
caregivers or family members as well. This index varies from zero to five.† Zero indicates independence while a maximum
score of five shows total dependence.
Table 3. Average
Dependence On Other Index In YOPD and General Onset PD:
††††††††††† Respondents Versus Hoehn and Yahr
Stage
The above table indicates that geriatrics in general serves as a
harbinger for dependence on others within a household.† Old age requires assistance without
Parkinsonís Disease and thus the General Onset respondentsí dependence on
others is skewed by the necessity for age based help.† It should be noted that even in the few years following YOPD
diagnosis or in the first two stages, dependence on others is above zero. This
means that men and women in their early 30ís and 40ís need assistance with
caring for themselves.† Upon realizing
that middle-aged respondents are unable to live without assistance, their
overall quality of life was scrutinized.†
The following table
compares the different components of the Quality of Life Index. †
Table 4. Comparison
of the Means of Quality of Life in YOPD Respondents and General Onset PD††
|
Quality of Life Components
|
In YOPD
Respondents
Mean
(Standard
Deviation)
|
In
General Onset
Respondents
Mean
(Standard
Deviation)
|
Population Mean
(Population
Standard Deviation)
|
|
Disease Severity
|
0.596
(0.320)
|
0.574
(0.283)
|
0.585
(0.298)
|
|
Degree of Depression
|
0.676
(0.475)
|
0.648
(0.401)
|
0.662
(0.433)
|
|
Disability
|
0.301
(0.406)
|
0.330
(0.377)
|
0.312
(0.386)
|
|
Cognitive Impairment
|
0.259
(0.310)
|
0.282
(0.372)
|
0.271
(0.344)
|
|
Psychological Effects
|
0.288
(0.346)
|
0.449
(0.394)
|
0.369
(0.370)
|
|
Quality of Life Index
|
2.120
(0.397)
|
2.283
(0.380)
|
2.202
(0.385)
|
†††††††††††
The table indicates an
overall lower quality of life in YOPD respondents. Note that the population
means and standard deviations are not known.†
Because of this, a two-sample t test statistic is performed and yields a
critical value of 1.8318.† When an
estimate of 35 is used as the degrees of freedom, P lies between 0.05 and
0.025.† The data strongly supports the
thesis that General Onset PD patients have a higher Quality of Life Index than
YOPD patients.† A 95% confidence
interval for the mean difference between YOPD respondents and General Onset
Parkinsonís Disease respondents uses the critical value t*=2.030 of the t(35) distribution.† The interval is 0.163Ī0.181 which
indicates that the General Onset Parkinsonís Disease respondents mean Quality
of Life Index is anywhere from -0.019 to 0.345 higher than the YOPD
respondentsí mean.† This data indicates
that roughly 90% of all tests will yield a higherQuality
of Life Index in General Onset PD respondents.†
Although the analysis does not solidify the difference between the two
diseases, it does indicate an unmistakable difference that requires further
questioning and investigation.
The breakdown of the
means must be analyzed while taking into account subjective shortcomings.† The statistics indicate that YOPD patients
have an overall lower quality of life than [that
of] General Onset Parkinsonís Disease patients.† It should be noted that Disease Severity
Index is based solely on the point of progression of the disease. If a perfect
sample size were taken, the Disease Severity Index between both groups would be
almost identical and YOPD respondents would have a comparatively lower mark
overall.† This, factored in with a
certain coefficient of geriatric effect on Parkinsonís Disease would more
evenly equate the two groups statistically.
The Quality of Life Index
also demonstrates that YOPD respondents suffer more from psychological effects,
disability, and cognitive impairment than General Onset respondents.† The increased suffering from disability and
cognitive impairment suggest that YOPD and General Onset PD are different. The
most common disability listed among YOPD respondents was the need for speech
therapy. In General Onset PD respondents, a cane was sited as the most commonly
needed aide.† Assuming that the two
diseases are in fact different, this sample indicates that the loss of voice
and speech patterns is more typical of YOPD than General Onset PD.† Cognitive Impairment was tested by asking
questions about problem solving, word retrieval, and short-term memory loss.†
One of the most common
signs of advanced aging is cognitive impairment.† For people over 50 years of age, cognitive impairment occurs
naturally.† This further illustrates the
difference in YOPD patients and General Onset Parkinsonís Disease patients.† Since cognitive impairment is aggravated by
geriatrics, General Onset PD patients should have an overall lower Quality of
Life component.† However, YOPD patients
are shown as being more heavily affected by cognitive impairment.†
The differences in the
two groupsí lifestyles must also be noted.†
While YOPD respondents show busy committed lives, General Onset
Parkinsonís Patients have much less to worry about.† Because stress and fatigue aggravate the symptoms of Parkinsonís
Disease, the YOPD patient normally suffers worse from his own lifestyle than
from the disease. The responsibility of the YOPD patient that comes with child
rearing and providing for a family brings about psychological issues more
often.† In this study, YOPD respondents
indicate a much higher frequency and more severe nature of mental illness.† It is necessary to note that the lifestyle
of a YOPD patient must be calmed after diagnosis.† In this way, psychological illnesses, faster disease progression,
and disability can be avoided.
The intention of this
research is to observe the behaviors and attitudes of both people with
Parkinsonís Disease and persons in the medical profession as well.† An analysis of the questionnaires has shown
a number of new significant findings that should be used to address the
attitudes of persons specializing in movement disorders.†
The most interesting of
the observations came while analyzing the Aggravating Factors portion of the
questionnaire.† In a study from
Neurology, pesticide exposure, family history of Parkinsonís Disease, and a
history of depression were sited as predictors for Parkinsonís Disease.
Exposure to pesticides and a rural living environment were considered the two
major causes of Parkinsonís Disease.†
The distinction in the two sets is that ďpredictorsĒ indicate pre-Parkinsonian
conditions that are indicated after Parkinsonís Disease is diagnosed, while
ďcausesĒ show conditions that have been scientifically proven to contribute to
the development of Parkinsonís Disease (Hubble et al. 1993). Much of the data
obtained from this study suggests different predictors for Parkinsonís
Disease.†
Of the seventy-eight
respondents, nineteen (nine YOPD and ten General Onset PD) reported some sort
of brain injury before diagnosis.† In
1996, an independent study found that approximately 2 million brain injuries. including
non-reported incidents. occurred in the US (Pallone 1996).† Of the 275 million people in the US, brain
injuries occur at about a rate of 0.72% of the population.† In the YOPD sample size, 26.4% of the
respondents reported some sort of brain injury before diagnosis.† Of the General Onset PD sample, 22.7% of the
respondents noted brain injury.† The
frequency of brain injury from this sample is not coincidentally high, but it
is significantly high.† This suggests a
link between head trauma and the development of Parkinsonís Disease.† Further studies should be performed in order
to specify the exact reason for such a high occurrence.
Only two of the 78
respondents were chronic cigarette users prior to diagnosis.† As compared to the national average of 26%,
a meager 2.5% of the sample of people with Parkinsonís Disease have smoked
(Wellner 2001).† Surprisingly, this
evidence seems unrealistic.† However, a
Cohort study done in a Californian retirement community showed that risk of PD
is significantly reduced by smoking, hypertension, coffee drinking, and alcohol
consumption.† The statistics in the
present study qualify the Cohort study in a random sampling from across the
country.
It has been generally
accepted that rural well water and prolonged pesticide exposure contribute to
the development of Parkinsonís Disease.†
But, of the 78 respondents, 70 reported living in an urban environment
for at least 16 years.† A startling 89%
of the sample size has been exposed to urban air pollution, drinking water, and
stress for at least a quarter of their lives.†
This generally contradicts the findings in the Hubble, et al.
study.† Further investigation must be
made in order to justify these results.
When asked which
medications bring about the most side effects, YOPD respondents mentioned
Sinemet as the medication.† Sinemet is a
dopaminergic and is used to replace or mimic the actions of dopamine (Johnson
1995). These YOPD respondents regularly listed side effects of Sinemet that
included light-headedness and fainting.†
These side effects are normally only attributed to dopamine agonists,
and not Sinemet.† From this observation,
it should be noted that YOPD respondents experience side effects from the most
common Parkinsonís Disease medication that people with Parkinsonís above the
age of 50 do not.
†††††††††
The intentions of this
study are to examine the human problems that patients have with Parkinsonís
Disease.† People with Parkinsonís
Disease are generally unsure of what effects the disease actually has on their
lives.† Also, the PD sufferer cannot
make distinctions between a medicationís shortcomings and the diseaseís
severity.† In an effort to sort out many
of these overlapping effects, this study has presented several viewpoints for
treatment.† Because of this different
perspective, subsequent studies should be performed in order to validate these
findings.† Subsequent research should be
tested with a larger sample size and a more concrete control group.†
When comparing the
diseaseís effect on a patientís quality of life, a holistic approach is
necessary.† The findings have
underscored psychological effects, disability, and cognitive impairment as the
strongest factors in lowering quality of life.†
Compared to the other factors taken into consideration in this study,
these three appear to be the most severe and life-altering effects of the
disease.† Since the YOPD patient scores
lower on the Quality of Life Index and suffers worse from these three factors,
the disease affects YOPD patients more severely.†
Due to the disease,
respondents have reported awkwardness with their spouse, inability to maintain
a household, and an overall feeling of helplessness. It can also be concluded
that General Onset Parkinsonís Disease patients suffer from denial and problems
with self-identity more so than the YOPD patient.† But, if YOPD patients are affected more drastically in the way
they think and act, then they are suffering from this disease more severely.† If Parkinsonís Disease changes the behavior
and lifestyle of younger patients so drastically as to make them doubt their
importance in a family, the younger patients have responded more severely to
the disease.†
Because the impact
Parkinsonís Disease has on YOPD patients is different from that of General Onset
Parkinsonís Disease patients, the groups must be suffering from two distinct
diseases.† If physiologically the
diseases are proven to be the same, then its psychological impact on the two
groups is indeed different.
Furukawa,
Yoshiaki, Yoshikuni Mizuno, and Hirotaro Narabayasi. ďEarly-Onset
Parkinsonism with Dystonia: Clinical Differences from Hereditary Progressive
Dystonia or Dopa-Responsive Dystonia.Ē Advances in Neurology. 39:327.
Goldfinger M.D., Stephen
E. ďA Special Report: Parkinsonís
Disease.Ē† Harvard Medical School
Health Publications Group.† Boston:
President and Fellows of Harvard College, 1998.
Hietanen, Marja, and
Heikk Teraevaeinen. ďThe
Effects of Age of Disease Onset on Neuropsychological Performance in
Parkinsonís Disease.Ē Journal of Neurology, Neurosurgery, and Psychiatry.
51(2) 2/1988: 244-249.
Hubble, Jean P., et al.† ďRisk
Factors for Parkinsonís Disease.Ē Neurology. 43(9) (9/1993):1693-1697.
Johnson, Arlette.† Young
Parkinsonís Handbook. APDA, 1995.
Kostic, Vladimir S., et
al.† ďEffects of Age at Onset on Frequency of Depression in
Parkinsonís Disease.Ē† Journal of
Neurology, Neurosurgery, and Psychiatry. 57(6) (10/1994): 1265-1267.
Lieberman, Abraham N. and
Frank L Williams. Parkinsonís
Disease.† New York: Simon and
Schuster, 1993.
Michaelson, Adam D. (2000-2001).†
[Interviews with Sydelle Lehrer and John Arnt].
Moskovitz, Charlene, et
al. ďLevadopa Induced Psychosis: A
Kindling Phenomenon.Ē† American† Journal of Psychiatry. 135(6) (6/1978):
669-675.
Nachmias, Chava and
David.† Research Methods in the Social Sciences.† New York: St. Martinís Press, 1992.
Nurmi, E, et al. ďRate of Progression in Parkinsonís Disease.Ē Journal
of the Movement Disorder† Society.† 16(4) (7/2001): 608-615.
Paganini-Hill, Annlia.† ďRisk
Factors for Parkinsonís Disease: The Leisure World Cohort Study.Ē
Neuroepidemiology. 20(2) (2001): 118-124.
Pallone, Frank Jr. ďProviding Expanded Studies and Innovative Programs
for Traumatic Brain Injury.Ē Congressional Record. Daily Edition. 142(100) (9
July 1996): H7126-7128.
Schrag, A., M.
Jahuppshahi, and N Quinn.† ďWhat Contributes to Quality of Life in
Patients with Parkinsonís Disease?Ē Journal of Neurology, Neurosurgery, and
Psychiatry. 69(9) (2000): 308-312.
Snyder, Solomon H., and
Robert J. DíAmato.† ďPredicting Parkinsonís Disease.Ē† Nature. 317(6034) (9/1985): 198-199.
Wellner, Alison Stein.
ďBlowiní Smoke.Ē† American Demographics. 23(2)
(2/2001): 20-21.